Rift Valley Fever

Human viral disease Rift Valley feverof tissue infected with Rift Valley fever virusSymptoms,Loss of sight, bleeding,DurationUp to a weekCausesspread by an infected animal orFinding or the virus in the bloodPreventionVaccinating animals against the disease, decreasing mosquito bitesTreatmentFrequencyOutbreaks in Africa andRift Valley fever ( RVF) is a that can cause mild to severe symptoms. The mild symptoms may include:, and which often last for up to a week. The severe symptoms may include: loss of sight beginning three weeks after the infection, infections of the causing severe headaches and, and bleeding together with which may occur within the first few days. Those who have bleeding have a chance of death as high as 50%.The disease is caused by the RVF, which is of the type. It is spread by either touching infected animal blood, breathing in the air around an infected animal being, drinking from an infected animal, or the bite of infected. Animals such as cows, sheep, goats, and camels may be affected.
Rift Valley fever (RVF) is a viral zoonosis that primarily affects animals but also has the capacity to infect humans. Nov 10, 2016 Rift Valley Fever Distribution Map; Countries reporting endemic disease and substantial outbreaks of RVF: Egypt, the Gambia, Kenya, Madagascar, Mauritania, Mozambique.
In these animals it is spread mostly by mosquitoes. It does not appear that one person can infect another person.
The disease is diagnosed by finding against the virus or the virus itself in the blood.Prevention of the disease in humans is accomplished by vaccinating animals against the disease. This must be done before an outbreak occurs because if it is done during an outbreak it may worsen the situation. Stopping the movement of animals during an outbreak may also be useful, as may decreasing mosquito numbers and avoiding their bites. There is a human; however, as of 2010 it is not widely available.
Mob wars cheats. It’s time to fulfill your family duty and live a life of crime in Mob Wars - La Cosa Nostra.
There is no specific treatment and medical efforts are supportive.of the disease have only occurred in Africa. Outbreaks usually occur during periods of increased rain which increase the number of mosquitoes. The disease was first reported among livestock in of in the early 1900s, and the virus was first isolated in 1931.
Contents.Signs and symptoms In humans, the virus can cause several syndromes. Usually, sufferers have either no symptoms or only a mild illness with fever, and abnormalities. In a small percentage of cases (.
Rift Valley fever phlebovirusThe virus belongs to the order. This is an order of enveloped negative single stranded RNA viruses. All Bunyaviruses have an outer lipid envelope with two —G(N) and G(C)—required for cell entry.
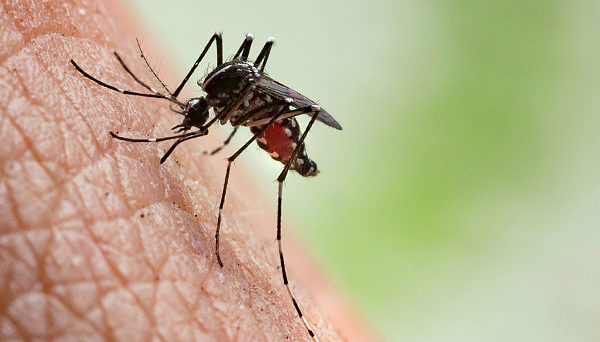
They deliver their genome into the host-cell by fusing their envelope with an.The virus' G(C) protein has a class II architecture similar to that found in. This structural similarity suggests that there may be a common origin for these viral families.The virus' 11.5 tripartite is composed of single-stranded. As a, it has an genome. Its L and M segments are negative-sense, but its S segment is ambisense. These three genome segments code for six major proteins: L protein , the two glycoproteins G(N) and G(C), the N protein, and the nonstructural NSs and NSm proteins.Transmission. See also:The virus is transmitted through mosquito, as well as through contact with the tissue of infected animals.
Two species— and —are known to transmit the virus. Other potential vectors include, Aedes mcintosh, Aedes ochraceus,. Contact with infected tissue is considered to be the main source of human infections. The virus has been isolated from two bat species: the ( Micropteropus pusillus) and the ( Hipposideros abae), which are believed to be for the virus. Pathogenesis Although many components of the RVFV's RNA play an important role in the virus’ pathology, the nonstructural protein encoded on the S segment (NSs) is the only component that has been found to directly affect the host. NSs is hostile and combative against the hosts interferon (IFNs) antiviral response. IFNs are essential in order for the immune system to fight off viral infections in a host.
This inhibitory mechanism is believed to be due to a number of reasons, the first being, competitive inhibition of the formation of the transcription factor. On this transcription factor, NSs interacts with and binds to a subunit that is needed for RNA polymerase I and II. This interaction cause competitive inhibition with another transcription factor component and prevents the assembly process of the transcription factor complex, which results in the suppression of the host antiviral response.
Transcription suppression is believed to be another mechanism of this inhibitory process. This occurs when an area of NSs interacts with and binds to the host's protein, SAP30 and forms a complex. This complex causes histone acetylation to regress, which is needed for transcriptional activation of the IFN promoter. This causes IFN expression to be obstructed. Lastly, NSs has also been known to affect regular activity of double-stranded RNA-dependent protein kinase R. This protein is involved in cellular antiviral responses in the host.
When RVFV is able to enter the hosts DNA, NSs forms a filamentous structure in the nucleus. This allows the virus to interact with specific areas of the hosts DNA that relates to segregation defects and induction of chromosome continuity. This increases host infectivity and decreases the host's antiviral response. Diagnosis Diagnosis relies on viral isolation from tissues, or serological testing with an.
Other methods of diagnosis include, and assays. As of September 2016, the (KEMRI) has developed a product called, designed to diagnose the disease in humans much faster than in previous methods.
Prevention A person's chances of becoming infected can be reduced by taking measures to decrease contact with blood, body fluids, or tissues of infected animals and protection against mosquitoes and other bloodsucking insects. Use of mosquito repellents and bed nets are two effective methods. For persons working with animals in RVF-endemic areas, wearing protective equipment to avoid any exposure to blood or tissues of animals that may potentially be infected is an important protective measure. Potentially, establishing environmental monitoring and case surveillance systems may aid in the prediction and control of future RVF outbreaks.No are currently available for humans. While a vaccines have been developed for humans, it has only been used experimentally for scientific personnel in high-risk environments. Trials of a number of vaccines, such as NDBR-103 and TSI-GSD 200, are ongoing. Different types of vaccines for veterinary use are available.
The killed vaccines are not practical in routine animal field vaccination because of the need of multiple injections. Live vaccines require a single injection but are known to cause birth defects and abortions in sheep and induce only low-level protection in cattle. The live-attenuated vaccine, MP-12, has demonstrated promising results in laboratory trials in domesticated animals, but more research is needed before the vaccine can be used in the field. The live-attenuated clone 13 vaccine was recently registered and used in South Africa. Alternative vaccines using molecular recombinant constructs are in development and show promising results.A vaccine has been conditionally approved for use in animals in the US. It has been shown that knockout of the NSs and NSm nonstructural proteins of this virus produces an effective vaccine in sheep as well.
Epidemiology.
Rift Valley fever, viral infection of animals that is transmissible to humans and causes a febrile of short duration. Headache, intolerance to light (photophobia), muscle pain, loss of appetite, and prostration are common symptoms. The virus is borne by and spread by the insect’s bite, although humans also can contract the disease by handling tissues or secretions of infected animals. The, first observed in the Rift Valley of, is found from through eastern and southern Africa.
Recovery from the fever is ordinarily uncomplicated; rarely, there may be, fatal hemorrhaging, or ocular involvement resulting in permanent visual impairment. There is no specific therapy. An strain of the causative virus has been used to immunize sheep and cattle, and a for humans has been under study.
Infamous 2 (stylized as inFAMOUS 2) is an action-adventure video game developed by Sucker Punch Productions and published by Sony Computer Entertainment for PlayStation 3 video game console. It is a sequel to the 2009 video game Infamous. Announced on June 4, 2010, the game was released on June 7, 2011. Infamous 2 walkthrough. InFamous 2 is the second chapter in the best-selling franchise for the PlayStation 3 from Sucker Punch. Infamous 2 (stylized as inFAMOUS 2) is an open-world action adventure game developed by Sucker Punch Productions and published by Sony Interactive Entertainment, released on June 7, 2011 in North America. It was also released on June 8 (Europe), June 9 (Australia), and July 7 (Japan). Infamous 2 is the second chapter in the best selling franchise for the PS3 system. This immersive open world action adventure offers a realistic take on being a super hero. Blamed for the destruction of Empire City and haunted by the ghosts of his past, Cole must make a dramatic journey to discover his full super-powered potential - and face the final confrontation with a dark and terrifying enemy from his own future. Infamous 2 opens in Empire City as a massive explosion rips through the town signaling the birth of a new evil. Nearly escaping death by the dark entity known as The Beast, Cole flees the city. Upon leaving, Cole discovers that The Beast has destroyed Empire City and is heading down the coast annihilating everything in its path. In an effort.